Our Viruses
Our Prototype Pathogen Approach prepares for potential outbreaks of taxonomically related viruses.
Our innovative and synergistic studies use a three-pronged strategy that blends: 1) structure-function investigation of surface proteins, 2) drivers of effective antibody and vaccines defenses against prototype viruses, and 3) systematic testing if defense strategies also work against related “test-case” viruses.
By pursuing these approaches in parallel for viruses in the family Paramyxoviridae and order Bunyavirales, we maximize synergistic returns and cross-talks between our projects. Learn more about our Prototype Pathogen Research Strategy here.
Paramyxoviridae Family
Paramyxoviruses cause respiratory infections in humans and animals. Many paramyxoviruses are highly transmissible. Scarce treatment and vaccine options for viruses in this family leave us vulnerable to outbreaks – especially if a Paramyxovirus that infects animals newly gains the ability to effectively infect humans.
Upon completion, we expect our studies to deliver antibody and vaccine candidates to prototype paramyxoviruses (PIV3, Mumps) that are accompanied by a deep-understanding of their workings that would enable adaptation to combat related newly-emerging Paramyxoviruses in an epidemic scenario.
Our work will also help address unmet clinical need against PIV3 and Mumps. PIV3 is a common respiratory infection of children, elderly and the immunocompromised, with no commercial vaccine available. Commercially licensed vaccines (MMR, MMRV) are available to protect against Mumps virus. However, the longevity of MMR and MMRV protection against Mumps is disappointing, with breakthrough infections common in adolescents and young adults.
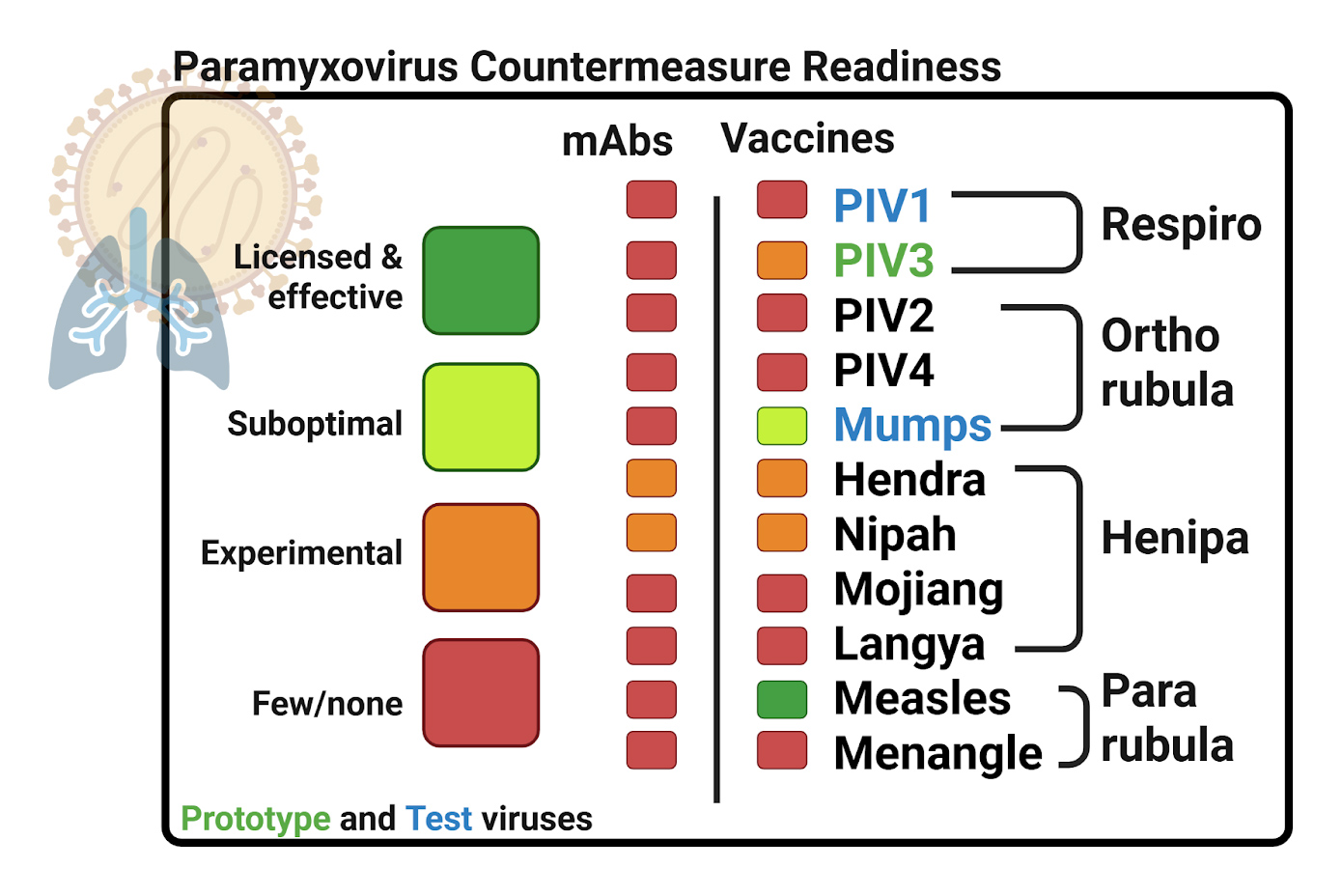
Respiroviruses
Respiroviruses are a subset of the Paramyxoviridae family and include the parainfluenza viruses 1 and 3 (PIV1, PIV3), and Sendai virus. Respiroviruses commonly infect children and currently lack any licensed vaccines or specific antiviral therapies.
Our prototype virus PIV3 is a common cause of respiratory infection in children and infants. Most cases of PIV3 resolve with supportive care, but a subset require interventions delivered in the hospital.
Rubulaviruses
Rubulaviruses include human parainfluenza virus 2 and 4 (HPIV1, HPIV4) and the Mumps virus. The licensed MMR and MMRV vaccines protect against mumps by providing a weakened Mumps virus version that stimulates immunity but does not cause disease. However, vaccine-induced immunity to Mumps is not long lasting, as evidenced by multiple outbreaks of Mumps among young adults. Interrogating how a vaccine could drive stronger and longer-lasting protection against Mumps is thus an opportunity to address unmet clinical need.
Bunyavirales Order
The Bunyavirales order includes many viruses capable of causing severe disease. Most are transmitted to humans from insect vectors. Spread of Bunyavirales insect vectors into new geographic regions, and sparse vaccine and anti-viral treatments for these viruses leave the world vulnerable to outbreaks.
We focus on the Prototype Viruses Oropouche, Rift Valley Fever Virus, and Toscana Virus, which have been responsible for outbreaks in Latin America, Africa, and Europe.
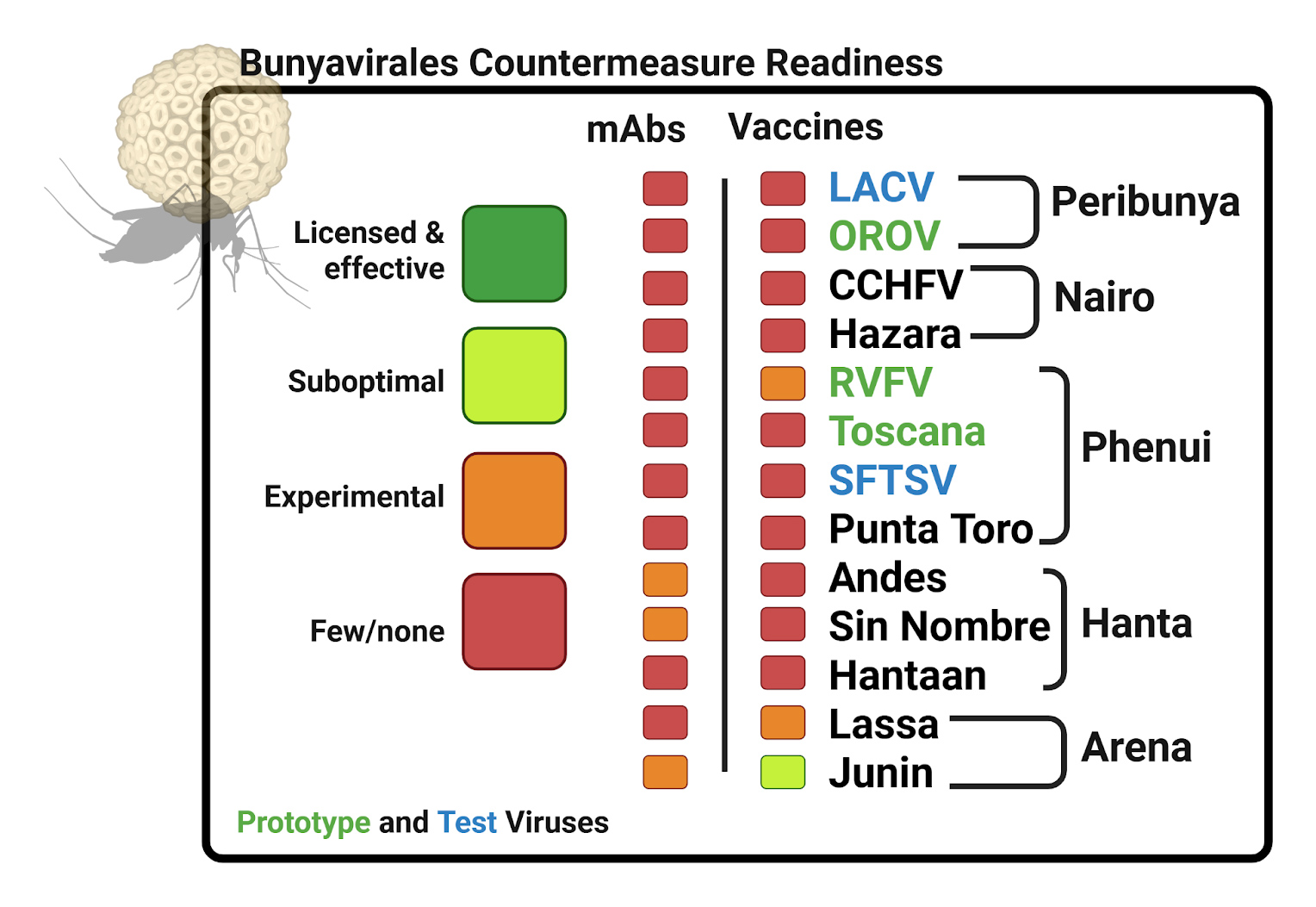
Perinbunyaviridae
Peribunyaviruses are viruses transmitted by arthropods, including mosquitoes and midges, and several are known to cause disease in humans. Among them, Oropouche virus (OROV) and La Crosse virus (LACV) are significant pathogens in the Americas.
Oropouche virus is a major cause of explosive febrile outbreaks in Central and South America and the Caribbean. The virus is primarily transmitted to humans by the bite of a tiny midge, Culicoides paraensis. OROV infection causes an acute, flu-like illness known as Oropouche fever, characterized by the sudden onset of high fever, headache, muscle and joint pain, and sometimes a rash. While most infections resolve within a week, a minority may develop neurological complications like meningitis. Recently, a new variant of the virus, established through a process called genetic reassortment, has fueled unprecedented outbreaks. This new lineage has spread far beyond its historical Amazonian range, causing widespread illness and its first-ever detection in countries such as Cuba. This has raised public health alerts, as the new variant is also being linked to more severe outcomes, including fatal cases and transmission from mother to fetus during pregnancy.
Phenuiviridae
Phenuiviruses are a diverse group of RNA viruses, many of which are spread by arthropods like mosquitos and sandflies. Some members can cause significant illness in humans and animals. Two important examples, Rift Valley fever virus (RVFV) and Toscana virus (TOSV), have distinct geographic ranges and different impacts on public health.
Rift Valley fever virus is mostly found in sub-Saharan African countries such as Kenya, South Africa, and Mauritania, but has also caused outbreaks in the Middle East, including Saudi Arabia and Yemen. The virus is primarily spread by mosquitoes, and is a major concern for both animal and human health. In livestock, RVFV can cause large outbreaks and significant economic loss. In people, it usually causes flu-like symptoms, but in some cases may progress to more severe illness, including hepatitis, hemorrhagic fever, or encephalitis. Human infections often follow outbreaks in animals and are linked to heavy rainfall and increased mosquito activity.
